
Deep-space astronomy sensor peers into the heart of an atom (Image Credit: Space.com)
Scientists have taken an instrument originally designed to study huge celestial objects in the cosmos and repurposed it to investigate the world on an infinitely smaller scale. With this instrument, they managed to probe the heart of the atom.
The team wanted to understand quantum-scale changes that occur within unstable atoms, and realized there’s a state-of-the-art gamma-ray polarimeter they could tap into. This device, known as a Compton camera, can measure the polarization of high-energy light waves. In other words, it can dissect what direction such high-energy light is orientated toward.
The only thing is, however, that this instrument was technically built for deep-space astronomy, not atomic investigations. In fact, scientists constructed it because they wanted to place it on the Hitomi satellite to make observations of high-energy cosmic processes. Yet, the camera has now proven its versatility. By capturing the polarization of gamma rays emitted from atomic nuclei rather than faraway galactic objects, it managed to reveal the internal structure of the atomic nucleus as well as any changes such nuclei may be undergoing.
Related: Atomic clocks on Earth could reveal secrets about dark matter across the universe
Compton chemistry 101
Compton cameras are used to determine the direction and energy of gamma rays using a phenomenon called “Compton scattering.”
Compton scattering happens when a high-energy particle of light, or “photon,” bounces off a charged particle, usually an electron. This interaction forces the photons hitting the electrons to “scatter,” meaning they transfer some of their energy and momentum to the particles they’ve just hit. In turn, those electrons can recoil and essentially pop off the atom they were previously attached to. This process can help reveal something about the atom that’s involved.
“The research team demonstrated that this Compton camera serves as an effective polarimeter for nuclear spectroscopy, revealing insights into the nuclear structure,” Tadayuki Takahashi, researcher leader and Kavli Institute for the Physics and Mathematics of the Universe scientist, told Space.com. “Developed initially for space observations, this instrument has now proven its worth as a tool for addressing complex scientific questions in other domains as well.”
The heart of an atom
You can think of atoms as composed of “shells.” Each shell is filled with varying portions of negatively charged electrons “buzzing” around; the outermost shell is known as the valence shell and the electrons within the valence shell are called valence electrons. These atomic shells surround a central nucleus comprised of positively charged protons and electrically neutral neutrons.
The number of protons in an atomic nucleus defines what element that atom represents.
For instance, hydrogen is the universe’s lightest element, and it always has one proton in its atomic nucleus. At the other end of the periodic table is uranium, one of the heaviest natural elements, which always has 92 protons in its nucleus. The number of neutrons in a nucleus doesn’t define what element an atom is, so it can vary. For instance, hydrogen can have no neutrons, one neutron in the case of deuterium, or two neutrons in the case of tritium. These atoms varying in weight, however, are called “isotopes.” Some isotopes are stable — others are not.
While 270 stable atomic nuclei are known to exist in nature, the number of known isotopes of elements jumps up to 3,000 when unstable atomic nuclei are factored in.
Interestingly, scientists have also recently observed phenomena associated with unstable atomic nuclei that aren’t seen around stable ones. These include anomalies in the electron energy levels as well as the disappearance and emergence of so-called “magic numbers.” Magic numbers refer to the amount of electrons it would take to fill those energy-level shells around an atomic nucleus. Conventionally, these numbers are 2, 8, 20, 28, 50, 82 and 126.
Thus far, however, conventional methods have been insufficient in investigating changes in nuclear structure related to these phenomena. This is due to the difficulty of balancing sensitivity and detection efficiency for instruments analyzing the characteristics of transitions undertaken by atoms.
Here’s lies the important part for the team’s investigation.
An unstable atomic nucleus will attempt to reach stability by ejecting a proton or a neutron. This is known as radioactive decay, and it’s a process that carries energy away from the atom in the form of photons. Gamma rays are a kind of photon — and the Compton camera can detect those gamma rays! Perhaps understanding the transition between instability and stability can help decode some of those weird atomic phenomena scientists have observed.

So, these researchers believed the Compton camera, which includes something called a Cadmium Telluride (CdTe) semiconductor imaging sensor, could be ideal for measuring the polarization of gamma rays from unstable nuclei. Again, this is because such a sensor offers high-detection efficiency and precise accuracy when determining the position of gamma rays (even though it was initially meant for deep-space gamma-ray signals).
The polarization of photons from charged particles turns unpolarized light into polarized light, with the orientation of polarization arising as a result of the scattering angle. The Compton camera can precisely measure this scattering angle and the polarization of these gamma rays, which indicates properties of particles within the atom, such as the value of quantum mechanical characteristics called “spin” and “parity.”
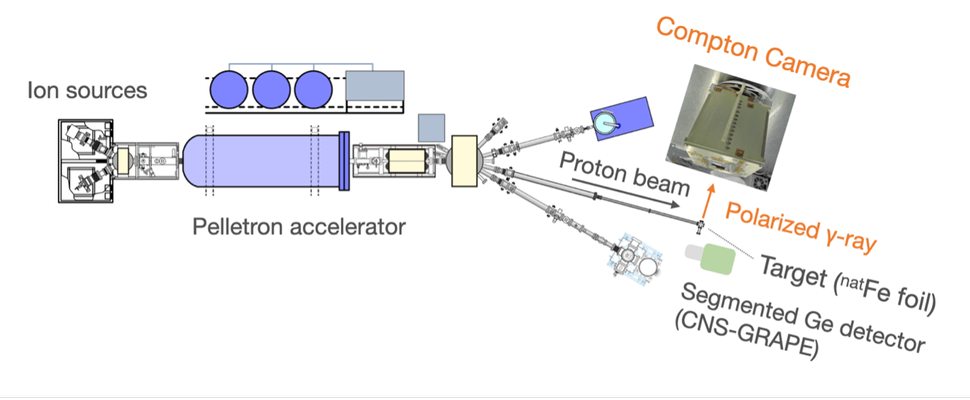
The scientists used accelerator experiments at the RIKEN research institute to perform a series of nuclear spectroscopy tests that involved blasting a film of iron nuclei with a beam of protons. This caused the electrons in the thin iron film to reach an excited state and emit gamma rays as they returned to their ground state. The team controlled both the position and intensity of these emissions artificially. This allowed for a detailed analysis of scattering events and the realization of a highly sensitive polarization measurement to test the capabilities of the Compton camera.
“The multi-layer CdTe Compton camera possesses several characteristics that make it well-suited for this research. First is the detection efficiency of CdTe,” Takahashi said. “Typically, gamma rays emitted from nuclei have energies in the order of Mega-electron Volt (MeV), where the detection efficiency for gamma-ray polarimeters tends to be low. However, the 20 layers of CdTe significantly enhance the efficiency of detecting these gamma rays.”
The Kavli Institute for the Physics and Mathematics of the Universe scientist added that the CdTe sensor developed by his group also achieves high-energy resolution for sub-MeV gamma rays.
“Lastly, it achieves a few millimeters of positional resolution within the detector’s effective area, enabling it to ‘see’ detailed Compton scattering patterns,” Takahashi added. “These patterns reflect the characteristics of the linear polarization of light, including gamma-rays.”
The emitted gamma rays were measured, revealing a peak structure, and the team was able to determine the angle at which photons were scattered. The team expected their results could be crucial for investigating the structure of rare radioactive nuclei, but even the lead researcher was surprised by just how successful this test was.
“The research group, comprised of experts in astronomical observation and nuclear physics, anticipated to some extent that gamma-ray polarimetry would be feasible for nuclear gamma-ray spectroscopy experiments,” Takahashi said. “However, the performance and results surpassed expectations.”
These experiments could be the tip of the iceberg when it comes to using space instruments to investigate atomic nuclei.
“There are various types of Compton cameras in astronomical observation, and they could be used similarly to measure the linear polarization of photons,” Takahashi concluded.
The team’s research is published in the journal Scientific Reports.





