Beyond Earth, the general scientific consensus is that the best place to search for evidence of extraterrestrial life is Mars. However, it is by no means the only place. Aside from the many extrasolar planets that have been designated as “potentially-habitable,” there are plenty of other candidates right here in our solar system. These include the many icy satellites that are thought to have interior oceans that could harbor life.
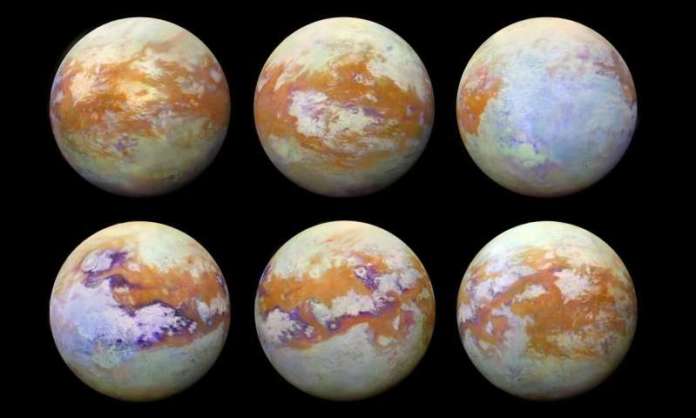
Among them is Titan, Saturn’s largest moon that has all kinds of organic chemistry taking place between its atmosphere and surface. For some time, scientists have suspected that the study of Titan’s atmosphere could yield vital clues to the early stages of the evolution of life on Earth. Thanks to new research led by tech-giant IBM, a team of researchers has managed to recreate atmospheric conditions on Titan in a laboratory.
Their research is described in a paper titled “Imaging Titan’s Organic Haze at Atomic Scale,” which recently appeared in the Feb. 12th issue of The Astrophysical Journal Letters. The research team was led by Dr. Fabian Schulz and Dr. Julien Maillard and included many colleagues from IBM Research-Zurich, the University of Paris-Saclay, the University of Rouen at Mont-Saint-Aignan, and Fritz Haber Institute of the Max Planck Society.
Much of what we know about Titan today is owed to the Cassini spacecraft, which orbited Saturn from 2004 to 2017 and finished its mission by diving into Saturn’s atmosphere. During this time, Cassini conducted many direct measurements of Titan’s atmosphere, revealing a surprisingly Earth-like environment. Basically, Titan is the only other body in the solar system that has a dense nitrogen atmosphere and organic processes taking place.
What is particularly interesting is the fact that scientists believe that roughly 2.8 billion years ago, Earth’s atmosphere may have been similar. This coincides with the Mesoarchean Era, a period where photosynthetic cyanobacteria created the first reef systems and slowly converted Earth’s atmospheric carbon dioxide to oxygen gas (eventually leading to its current balance of nitrogen and oxygen).
While the surface of Titan is believed to hold clues that could improve our understanding of how life emerged in our solar system, getting a clear look at that surface has been a problem. The reason for this has to do with Titan’s atmosphere, which is permeated by a dense photochemical haze that scatters light. As Leo Gross and Nathalie Carrasco (co-authors on the study) explained in recent article posted to the IBM Research Blog:
“Titan’s haze consists of nanoparticles made of a wide variety of large and complex organic molecules containing carbon, hydrogen and nitrogen. These molecules form in a cascade of chemical reactions when (ultraviolet and cosmic) radiation hits the mix of methane, nitrogen and other gases in atmospheres like Titan’s.”
As a result, there is still much that scientists don’t know about the processes that drive Titan’s atmosphere, which includes the exact chemical structure of the large molecules that make up this haze. For decades, astrochemists have been conducting laboratory experiments with similar organic molecules known as tholins–a term derived from the Greek word for “muddy” (or “hazy”).
Tholins refer to a wide variety of organic carbon-containing compounds that form when exposed to solar UV or cosmic rays. These molecules are common in the outer solar system and are typically found in icy bodies, where the surface layer contains methane ice that is exposed to radiation. Their presence is indicated by surface that have a ruddy appearance, or like they have sepia-colored stains.
For the sake of their study, the team led by Schulz and Maillard conducted a experiment where they observed tholins in various stages of formation in a laboratory environment. As Gross and Carrasco explained:
“We flooded a stainless-steel vessel with a mixture of methane and nitrogen and then triggered chemical reactions through an electric discharge, thereby mimicking the conditions in Titan’s atmosphere. We then analyzed over 100 resulting molecules composing Titan’s tholins in our lab at Zurich, obtaining atomic resolution images of around a dozen of them with our home-built low-temperature atomic force microscope.”
By resolving molecules of different sizes, the team was provided with glimpses of the different stages through which these haze molecules grow, as well as what their chemical makeup looks like. In essence, they observed a key component in Titan’s atmosphere as it formed and accumulated to create Titan’s famous hazy effect. Said Conor A. Nixon, a researcher with NASA’s Goddard Space Flight Center (who was not affiliated with the study): “This paper shows ground-breaking new work in the use of atomic-scale microscopy to investigate the structures of complex, multi-ringed organic molecules. Typical analysis of laboratory-generated compounds using techniques such as mass spectroscopy reveals the relative proportions of the various elements, but not the chemical bonding and structure.
“For this first time here, we see the molecular architecture of synthetic compounds similar to those thought to cause the orange haze of Titan’s atmosphere. This application now provides an exciting new tool for sample analysis of astrobiological materials, including meteorites and returned samples from planetary bodies.”
What’s more, their results may also shed light on Titan’s mysterious methane-based hydrological cycle. On Earth, this cycle consists of water transitioning between a gaseous state (water vapor) and a liquid state (rain and surface water). On Titan, the same cycle takes place with methane, which transitions from atmospheric methane gas and falls as methane rain to form Titan’s famous hydrocarbon lakes.
In this case, the research team’s results could reveal the role that the chemical haze plays in Titan’s methane cycle, including whether or not these nanoparticles can float on its methane lakes. Furthermore, these findings could reveal whether or not similar atmospheric aerosols helped life emerge on Earth billions of years ago.
“The molecular structures we have now imaged are known to be good absorbers of ultraviolet light,” described Gross and Carrasco. “That, in turn, means that the haze may have acted as a shield protecting DNA molecules on the early Earth’s surface from damaging radiation.”
If this theory is correct, the team’s findings would not only help scientists to understand the conditions under which life emerged here on Earth, they could also point towards the possible existence of life on Titan. The mysterious nature of this satellite is something scientists first became aware of in the early 1980s, when the Voyager 1 and 2 space probes both flew through the Saturn system. Since then, scientists have pieced together
By the 2030s, NASA plans to send a robotic rotorcraft called Dragonfly to Titan to explore its surface and atmosphere and search for possible signs of life. As always, the theoretical work and laboratory experiments performed in the meantime will allow scientists to narrow the focus and increase the odds that the mission (once it arrives) will find what its looking for.
Explore further
Fabian Schulz et al. Imaging Titan’s Organic Haze at Atomic Scale, The Astrophysical Journal (2021). DOI: 10.3847/2041-8213/abd93e
Journal information:
Astrophysical Journal
,
Astrophysical Journal Letters
Source
Universe Today
– Advertisement –