Moon rocks aren’t just about the rock — the gas trapped inside is just as intriguing.
A new examination of six lunar meteorites found in Antarctica has revealed the first definitive proof that the moon inherited chemical elements from Earth’s interior. The discovery adds support to the theory that our planet’s most enduring companion was born when something massive slammed into the Earth in the distant past, also known as the giant impact theory.
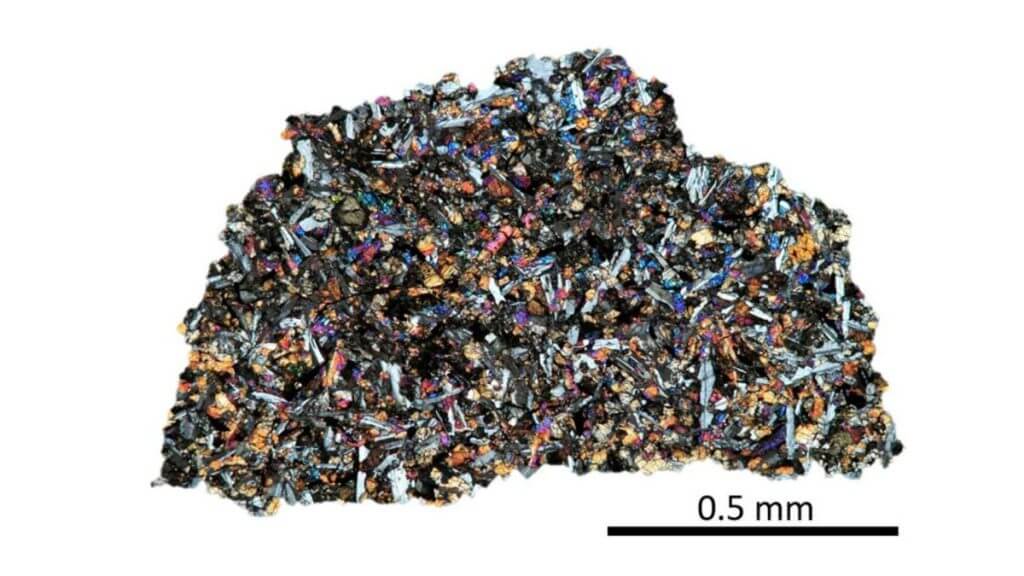
During doctoral research at ETH Zurich in Switzerland, Patrizia Will discovered traces of helium and neon — both noble gases, which rarely bond to other elements — in six lunar meteorites from NASA’s Antarctic collection.
Related: The moon dazzles in photo from International Space Station
The meteorites are composed of volcanic rock called basalt that formed as magma welled up from the interior of the moon then rapidly cooled. This cooling process created lunar glass particles within the samples that retain chemical signatures of solar gases. After the basalt formed, additional layers of rock enveloped it, protecting the glass from charged particles, both those from the sun’s constant stream of solar wind and those from beyond the solar system, dubbed cosmic rays. The isolation preserved this fingerprint and guaranteed the origin of the gases trapped inside, the researchers reasoned.
Scientists were able to catch the fingerprints of helium and neon in the meteorites thanks to a particularly sensitive noble gas mass spectrometer that researchers nicknamed Tom Dooley after a Grateful Dead song. (Mass spectrometers sort out by weight elements within a sample.)
“Finding solar gases, for the first time, in basaltic materials from the moon that are unrelated to any exposure on the lunar surface was such an exciting result,” Will said in a statement.
Lunar research with impact
The finding supports the idea that a giant impact created the moon, and the work could also lay a roadmap for research into how the solar system’s rocky worlds formed.
One version of the giant impact theory proposes that a protoplanet called Theia smashed into Earth around 4.5 billion years ago, about 60 million years after the Earth itself formed.
The impact must have been truly massive to throw out ejecta from Earth’s interior that was able to remain in orbit and coalesce into another body, rather than falling back down to our infant planet.
Other lines of evidence that support this theory include the fact that the moon is lightweight, lacking large amounts of iron in its interior, while 30% or so of Earth’s mass is sealed in its iron-rich core. The moon’s mantle rocks also have a similar composition to those of Earth, and these rocks are both significantly different from Martian meteorites.
Scientists needed a somewhat smaller impact to conduct the study. With no dense atmosphere like Earth’s to burn up space rocks, the moon is constantly bombarded by asteroids. It was probably a high-energy impact from just such an asteroid that dug out rock fragments from the ejected rock fragments from deep within a large lava flow on the moon. These fragments eventually fell to Earth as meteorites; scientists spotted the dark space rocks against the blinding white of Antarctica.
The researchers hope that scientists’ understanding of far more than the moon could benefit from the team’s work, since the current analysis targeted just a few of NASA’s collection of around 70,000 meteorites.
“I am strongly convinced that there will be a race to study heavy noble gases and isotopes in meteoritic materials,” Henner Busemann, a geochemist at ETH Zurich, said in the same statement. He thinks that researchers will soon be looking to meteorites to find other noble gases like xenon and krypton that are more challenging to identify than helium and neon.
“While such gases are not necessary for life, it would be interesting to know how some of these noble gases survived the brutal and violent formation of the moon,” Busemann said. “Such knowledge might help scientists in geochemistry and geophysics to create new models that show more generally how such most volatile elements can survive planet formation, in our solar system and beyond.”
The team’s findings are discussed in a paper (opens in new tab) published Wednesday (Aug. 10) in the journal Science Advances.
Follow us on Twitter @Spacedotcom and on Facebook.